- NEWS
- the EDIT
- COMMENTARY
- BUSINESS
- LIFE
- SHOW
- ACTION
- GLOBAL GOALS
- SNAPS
- DYARYO TIRADA
- MORE

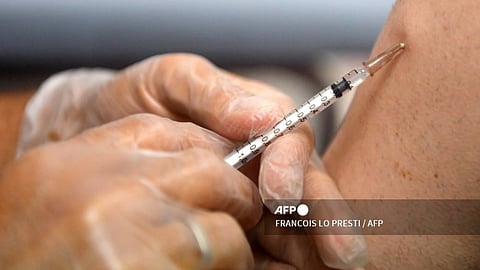
The Food and Drug Administration (FDA) on Tuesday announced the approval of GSK Philippines’ Meningococcal Group B Vaccine for the prevention of invasive meningococcal disease (IMD) in individuals aged two months and older.
IMD is a serious infection caused by the bacterium Neisseria meningitidis, which can lead to life-threatening conditions such as meningitis, an inflammation of the membranes surrounding the brain and spinal cord and meningococcal sepsis, where the infection spreads into the bloodstream.
Both conditions require urgent medical attention to prevent serious and irreversible consequences, including long-term complications or death.
Neisseria Meningitidis has 13 serogroups, of which A, B, C, W, X and Y are the most important medically, with serogroup B being one of the primary causes of invasive meningococcal disease globally and in the Philippines.
Prior to this FDA approval, there was no vaccine available for serogroup B.
